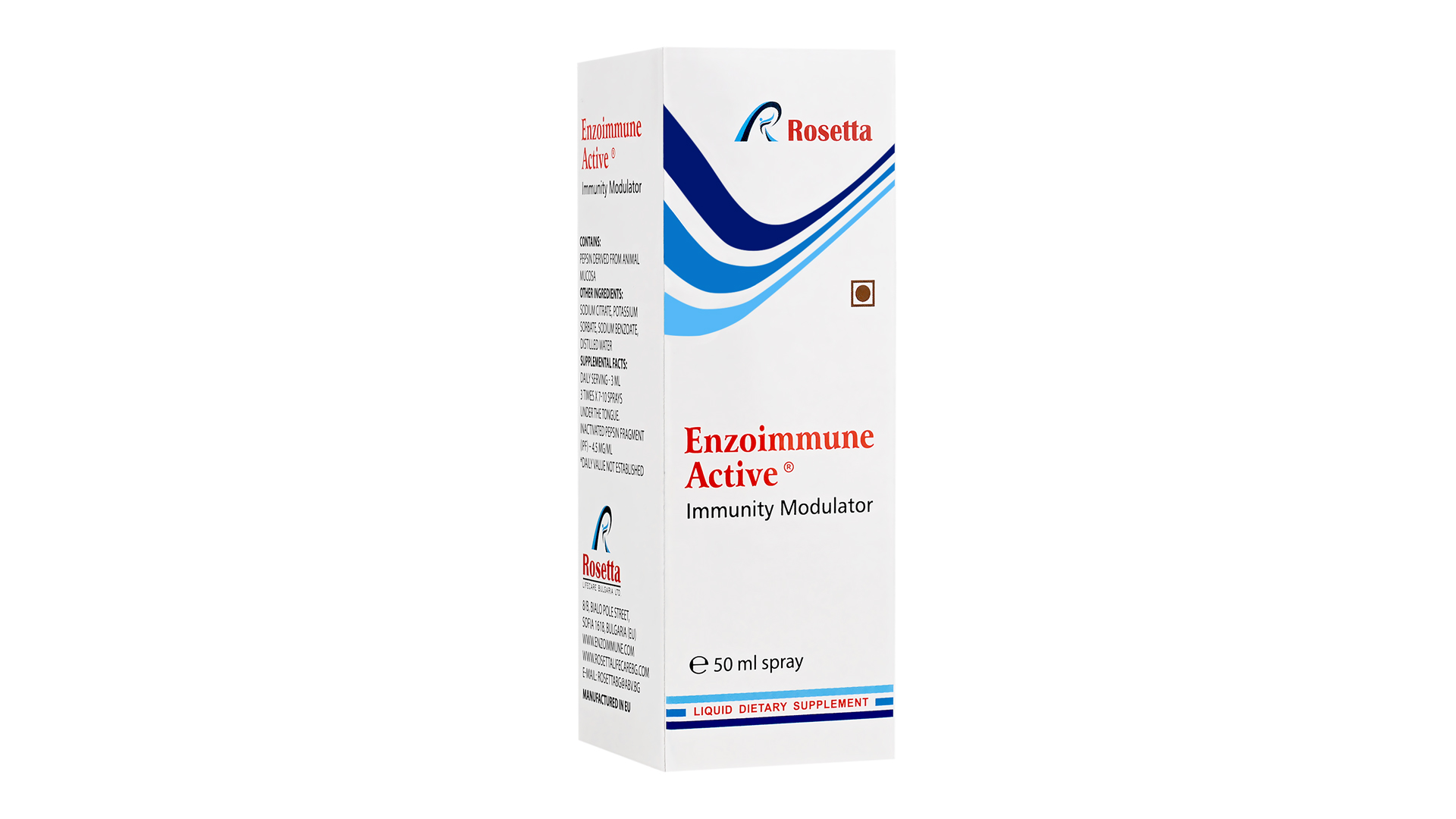
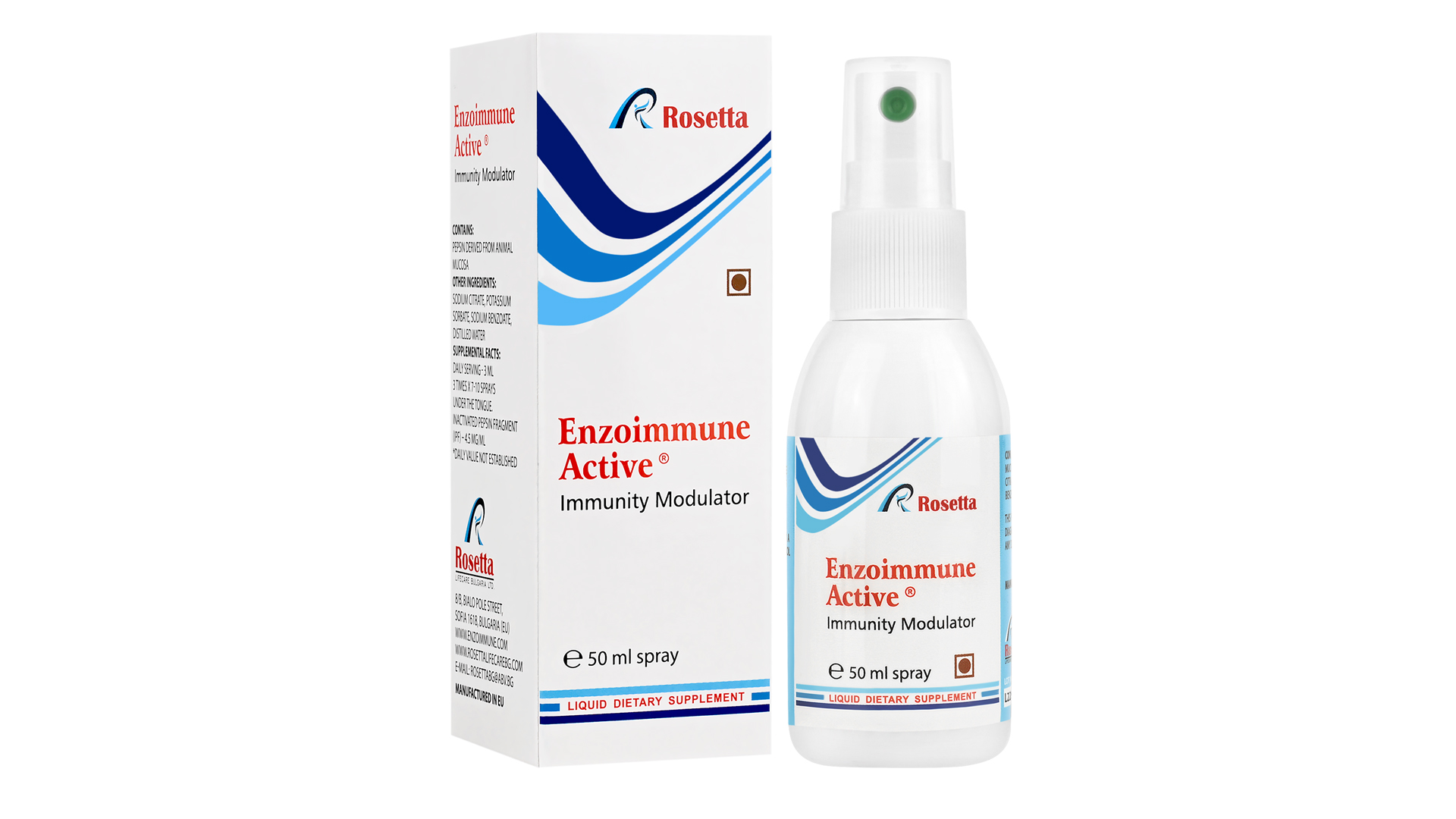
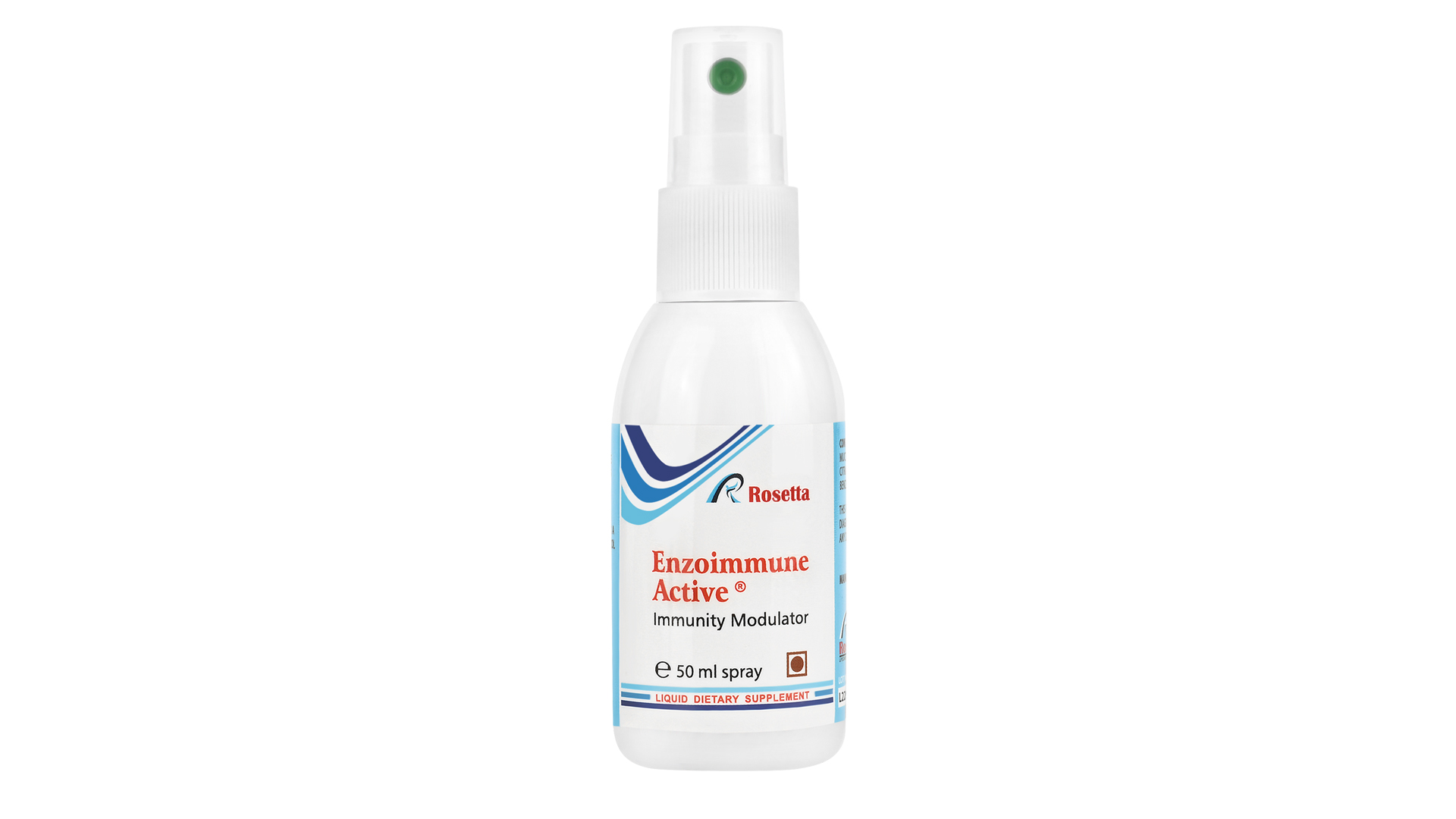
Enzoimmune Active Liquid Food Supplement to Boost Immunity
$ 28
Our immunomodulator is a dietary supplement that acts favorably on the immune system by enhancing its ability to fight infections, supporting the formation of antibodies, and contributing to the body’s antioxidant defense. It has a direct antiviral effect, limiting viral replication and reducing the viral load. It is highly effective in alleviating autoimmune diseases such as psoriasis and asthma, but also other complicated medical conditions – as a helping part of more complex therapy.
In stock